
/cloudfront-us-east-1.images.arcpublishing.com/gray/VI3NHOJIMBBGTLHYK4N3SHBPVU.jpg)

The company has posted a list of devices covered by the recall. The recall notes that the foam may degrade over time and may also emit at least two harmful toxins. Philips said it will replace the current foam with a new material and obtain the relevant regulatory clearances. On June 14, Philips issued a recall for many of its CPAP, BiPAP and ventilator machines because of concerns that users may inhale small particles of the foam used to dampen sound while the machines are in use. Per the agency, a recall is "a method of removing or correcting products that are in violation of laws administered by the Food and Drug Administration." Class I recalls are the most serious type of recall for products. The FDA classified the Philips CPAP recall as a Class I recall in July 2021. V60 ventilators upgraded to enable High Flow Therapy (software version 3.00 and 3. Philips CPAP machines were recalled because polyester-based polyurethane (PE-PUR) used to control sound and vibration in some CPAP, BiPAP and ventilator machines may break down and release toxic particles and gasses that users may inhale or swallow. The recall affects 2 ventilator models sold between May 1, 2009, and June 2, 2021: 1. The Philips' recall of sleep apnea machines and respiratory care devices is considered a voluntary recall, as are most recalls of medical devices, according to the Food and Drug Administration. Philips Respironics has recalled more than 16,000 ventilators in the United States due to a problem with the machines oxygen flow. Those who are using a life-sustaining affected device shouldn't stop prescribed therapy, Philips said, until consulting a physician. Philips hasn't reported any deaths associated with its recalled devices, but the company said those with a Bi-Level PAP or CPAP device should discontinue use and consult with a health care provider to determine the most appropriate options for continued treatment. Chemical exposure due to off-gassing can cause many of the same symptoms, the company said, along with hypersensitivity, nausea and vomiting.

Please note that delays are to be expected as some replacements are currently out of stock.The exposure to foam particles could cause headache, irritation, inflammation, respiratory issues, and possible toxic and carcinogenic effects, Philips said. It is possible that the waiting time will be longer than usual Currently we are receiving a high number of calls and emails, we will make sure to respond to you as soon as possible. This situation is beyond our control, however we try to do our best to support you in these circumstances. The decision to continue therapy or to discontinue it while the devices are being replaced is up to you and your doctor. We understand that each case is unique, so we invite you to contact your doctor to decide the best solution for you and to follow up on your therapy. Número do SAC: 08 de Segunda a sexta das 8h às 20h. Conforme sejam viabilizadas soluções adicionais, atualizaremos nosso SAC e site exclusivos do recall.
Philips recall serial number#
In addition, we will provide the serial number of your device to the manufacturer. A Philips está trabalhando arduamente na tentativa de antecipar este prazo. We are committed to keeping you informed as soon as we learn of the complete procedure issued by Philips. The Sommeil Blue team is aware that trust is the foundation of our ongoing partnership in your care and especially in your therapeutic follow-up, and we strive to maintain this trust every day.
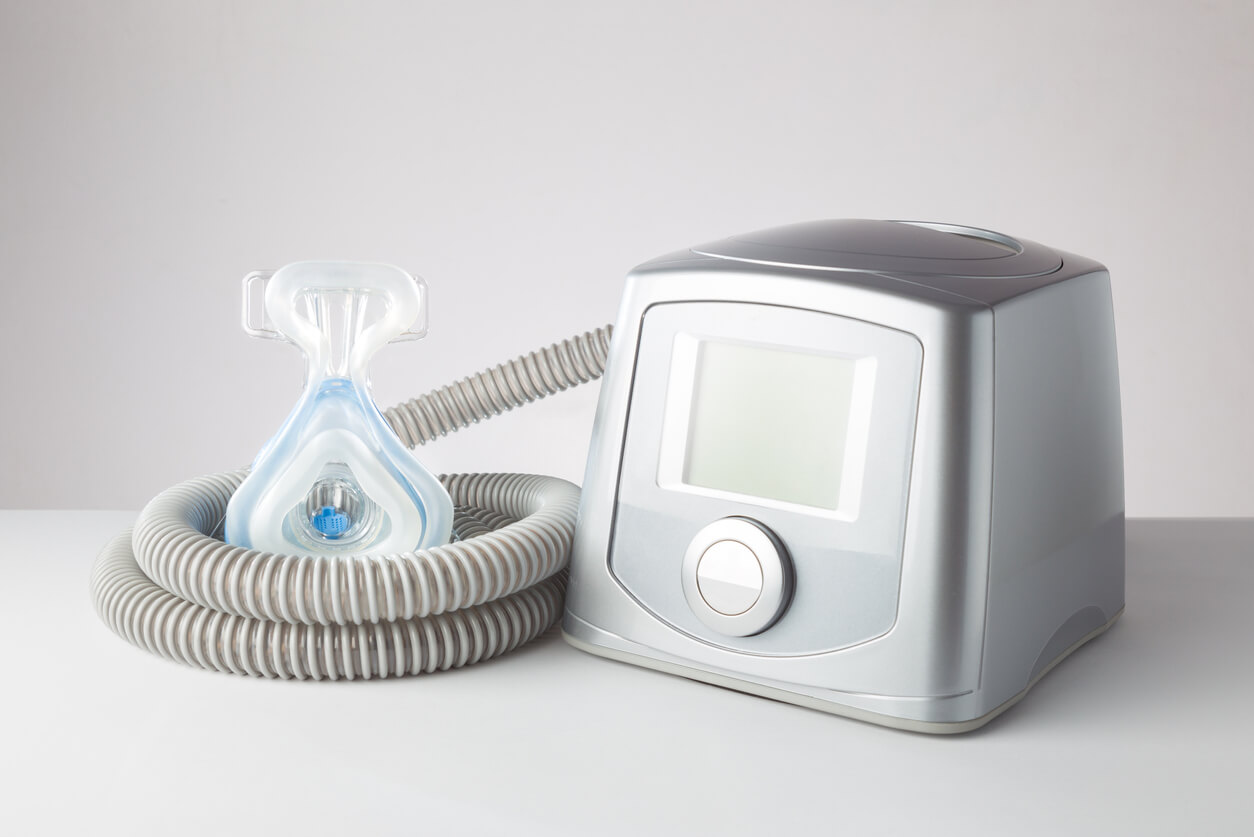
You can find the recall announcement of Philips recommendations here. Philips Respironics has announced a voluntary recall of continuous and non-continuous ventilators (CPAP, Travel CPAP and Bipap) due to two problems with the polyester-based polyurethane (PE-PUR) sound deadening foam used in these devices that can degrade and be ingested or inhaled, the problem is reported at 0.03%.


 0 kommentar(er)
0 kommentar(er)
